Using Mass Describe the Relative Size of the Subatomic Particles
A photon is an. K-and K have a PDG rest mass value of 04937 GeV.

Atomic Structure And Subatomic Particles Youtube
Two are charged particles K-and K and one is a neutral particle K0.
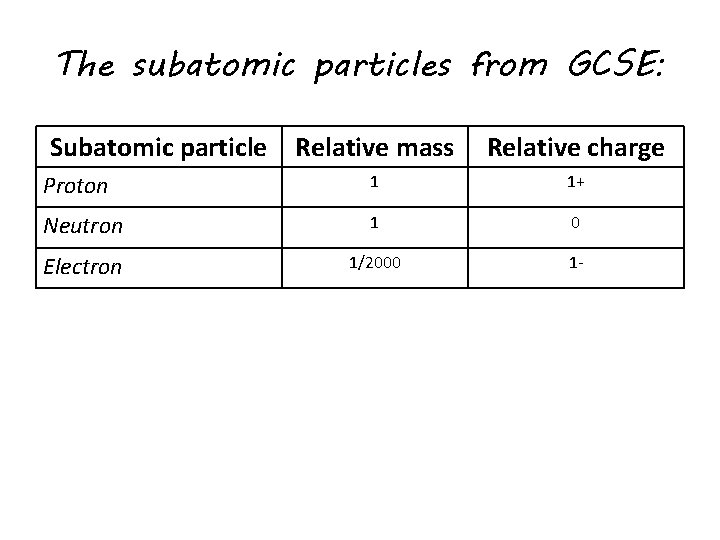
. 1 relative positive charge 160 10¹⁹ C. Like other charged particles the difference in rest mass value fits within. Subatomic particle Relative mass Relative charge.
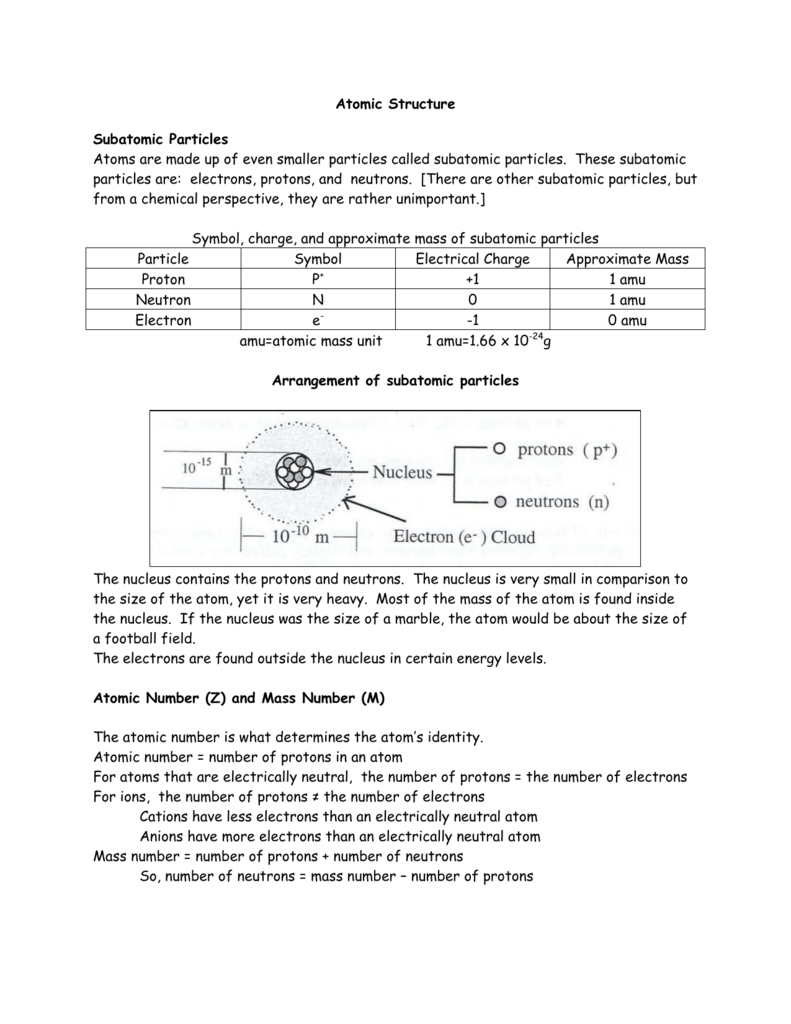
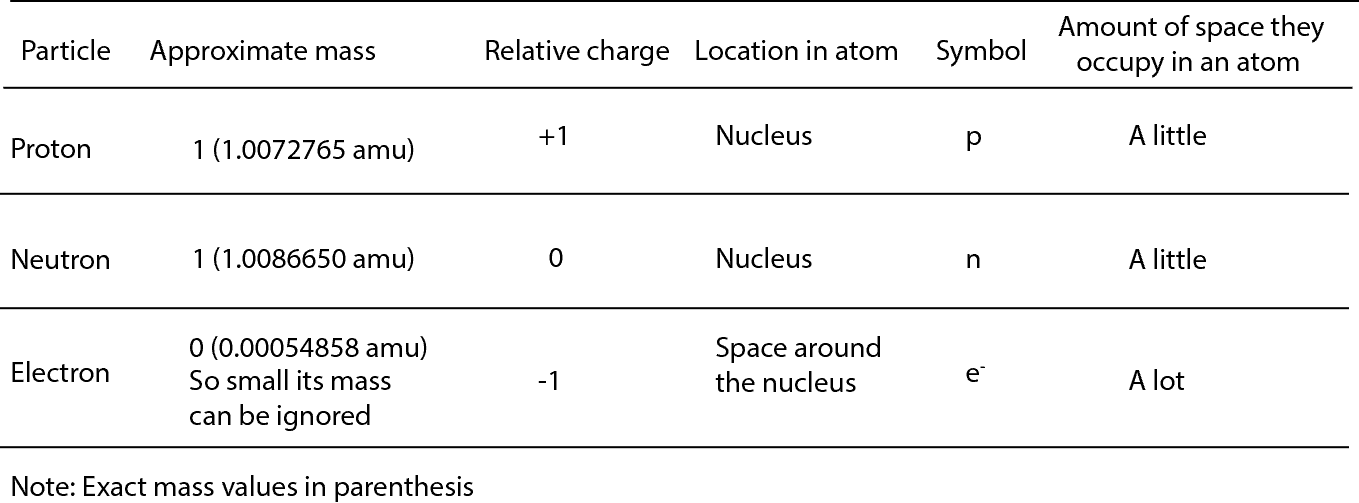
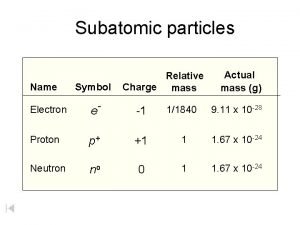
1 relative mass 166 10²⁴ g. List the subatomic particles and describe their relative masses charges and positions in the atom Atom. The mass of a proton is about 1800 times greater than the mass of an electron.
Its very similar but slightly larger certainly more massive. Provide students with information regarding the relative mass of the proton and the electron. You a neutron has a mass of 1675 times 10 to the negative 27 kg also about one AM you.
A proton has a mass of 16726 x 10-24grams. Describe the relative size location and electrical charge of protons neutrons and electrons with an atom. 1 relative negative charge -160 10¹⁹ C.
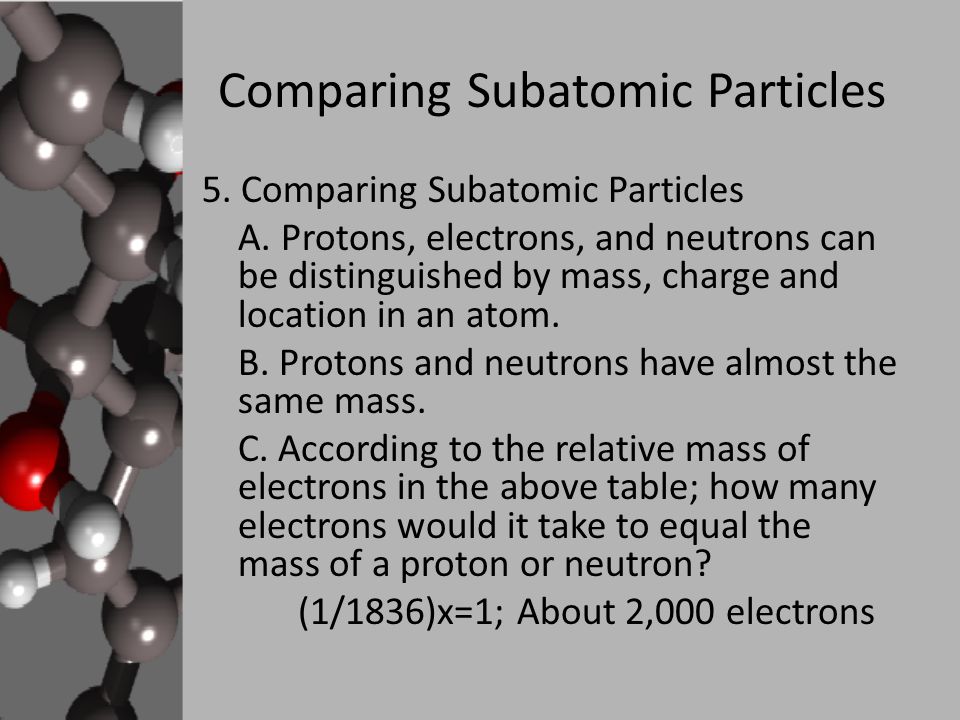
Then tell students that the distance of the electron from the. Explain how the atomic number and mass number are determined. The relative mass of a proton is 1 and a particle with a relative mass smaller than 1 has less mass.
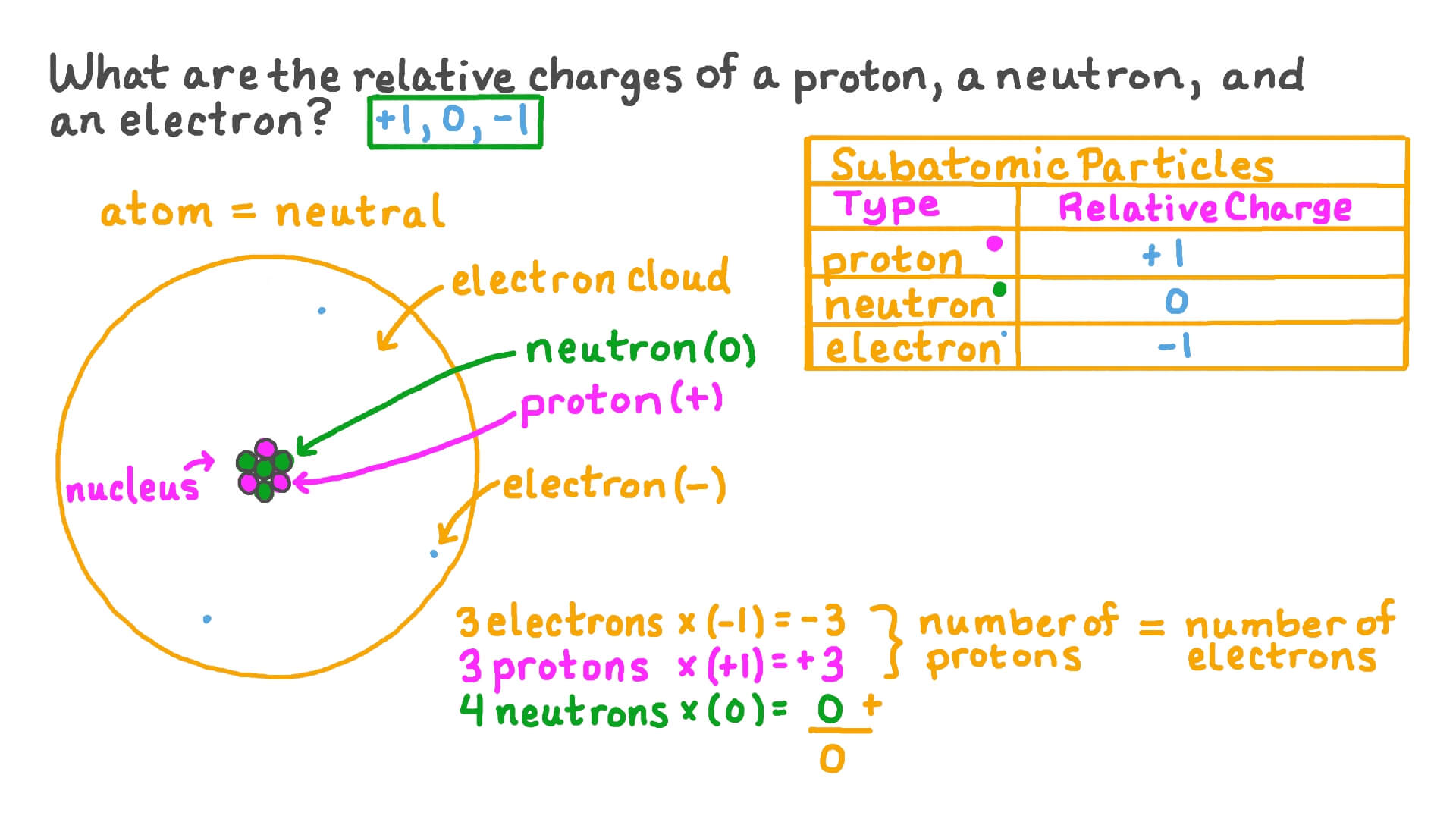
And so if we look at their relative mans we make it relative to the proton. A proton has a mass of 167 3 times 10 to the -27 kg. Atoms are composed of subatomic particles.
A proton is a subatomic particle with a single unit of positive electrical charge. An electron has a relative charge of -1602 x 10-10. The two types of subatomic particle are elementary they are not made of other particles and composite.
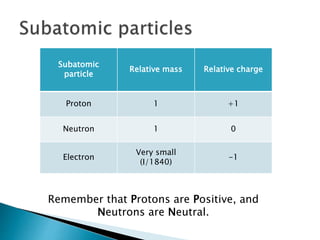
So lets list these subatomic particles their mass and their charge. The relative charge of protons is 1 the relative mass of a proton is 1 and a proton is located in the center of the atomnucleus. Protons are practically the same size as neutrons and both are much larger than electrons.
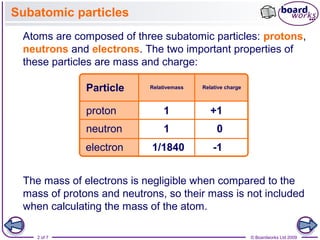
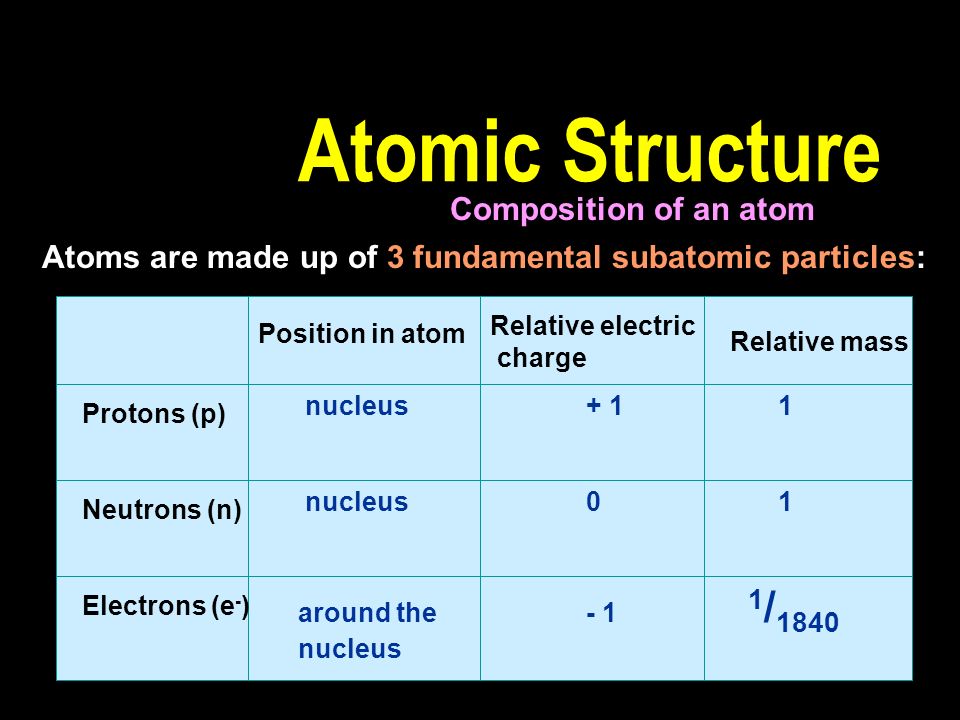
A proton has a mass approximately 1836 times greater than the mass of the electron but the masses of protons and neutrons differ less than one percent. K0 has a PDG rest mass value of 04976 GeV. Subatomic particle Relative mass Relative charge Proton 1 1 Neutron 1 0 Electron 00005 -1.
There are three kaons K. At 1675 times tended mice 24 grands and the election is much smaller at 911 times 10 to the minus 28 grams. A subatomic particle are smaller particles composing nucleons and atoms.
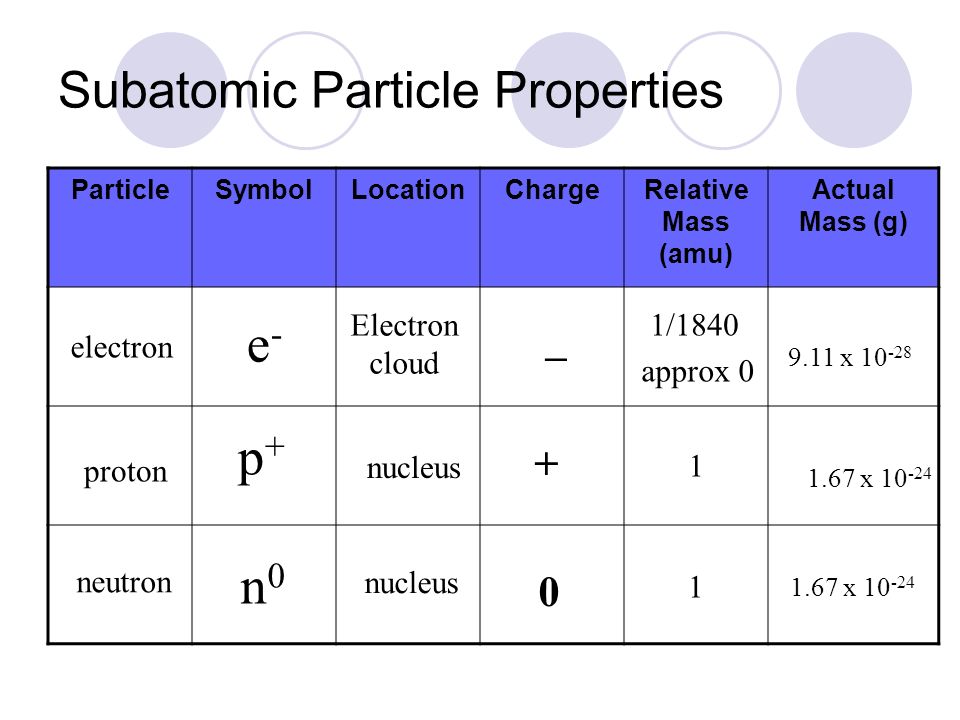
Properties of Subatomic Particles Particle Charge Relative mass Proton 160 x 10-19 C 1 amu Electron -160 x 10-19 C 0 amu 11840 amu Neutron neutral 1 amu. E3radg8 and 1 more users found this answer helpful. A proton has a relative charge of 1602 x 10-19 Coulomb and a mass of 1672 x 10-24 gram.
Atoms - Molecules - Ions Particle Mass kg Mass amu Charge Electron 910939 x 10 -31 000055 -1 Proton 167262 x 10 -27 100728 1 Neutron 167262 x 10 -27 100728 0 Most of the mass in an atom is in the nucleus Subatomic Particles. The mass of electrons is very small compared to protons and neutrons. The building blocks of elements Proton.
The subatomic particles of atoms are Protons Neutrons. Grams g and coulombs C are the units of absolute masses and absolute charges respectively. But you will notice that the mass of a neutron although comparable almost exactly the same as that of a proton is slightly larger.
So the mess the absolute mass for Proton is 1673 times 10 to the negative 24 grams from the neutron. The rest masses are slightly different which is explained below. The relative charge of the neutron is 0 the relative mass of a neutron is 1 and the neutron is located in the center of atomsnucleus along with protons.
Protons electrons and neutrons. Positive charge found in nucleus 1008 amu. Which option correctly describes the relative charges and masses of the subatomic particles.

2 1 Relative Masses Charges And Positions Of The Subatomic Particles Sl Ib Chemistry Youtube
Atomic Structure Subatomic Particles Atoms Are Made Up Of Even

Size Mass Of Atoms Electrical Charges Of Subatomic Particles Aqa C1 4 1 New Spec 9 1 2018 Teaching Resources

Solved Model 1 Subatomic Particles Relative Mass Atomic Chegg Com

Atoms And Subatomic Particles Exit Ticket Assessment Exit Tickets Atom Particles

Matter Atoms Quarks Leptons Fizik Ve Matematik Kuantum Mekanigi Fizik Bilimi
Atomic Structure Part 1 Fundamental Particles
I Subatomic Particles P Ppt Video Online Download

1 Atomic Structure Objectives Describe Properties Of Subatomic
What Re The Properties Of The Subatomic Particles And How Are These Particles Related
What Are Relative Charge And Relative Mass Quora
Question Video Identifying The Relative Charge Values Of Subatomic Particles Nagwa

Atomic Structure Pbs Learningmedia Resource Classroom Matter Science Pbs Learning Media
Atomic Structure Composition Of An Atom Atoms Are Made Up Of 3 Fundamental Subatomic Particles Relative Mass Relative Electric Charge Position In Atom Ppt Download

Question Video Arranging Subatomic Particles According To Their Masses Nagwa
The Structure Of An Atom Ppt Download
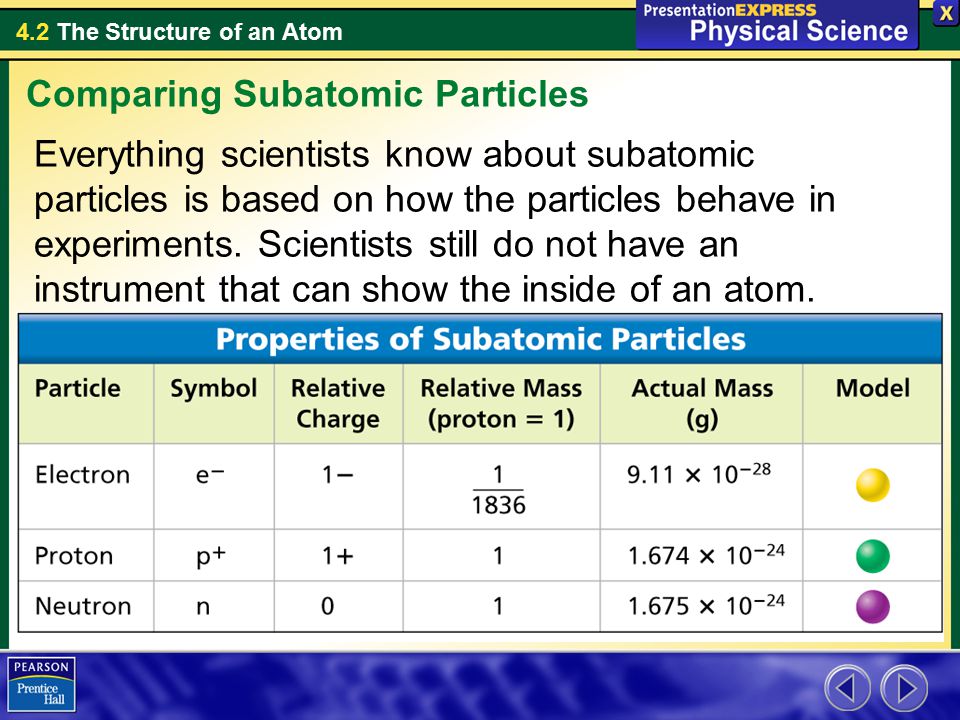
Properties Of Subatomic Particles Protons Electrons Neutrons Ppt Video Online Download
Comments
Post a Comment